Technical Due Diligence & Evidence Strategy for Global Biotech Value Realization
F E A T U R E S
Bridging the gap between scientific innovation and commercial value. Headquartered in Geneva.
In the 2026 JCA landscape, traditional due diligence is insufficient. At Sur Access, we don't just 'assess information'; we perform Technical Stress Tests on clinical protocols to verify that your asset’s reimbursement potential and price floors are anchored in evidence—not just spreadsheets.
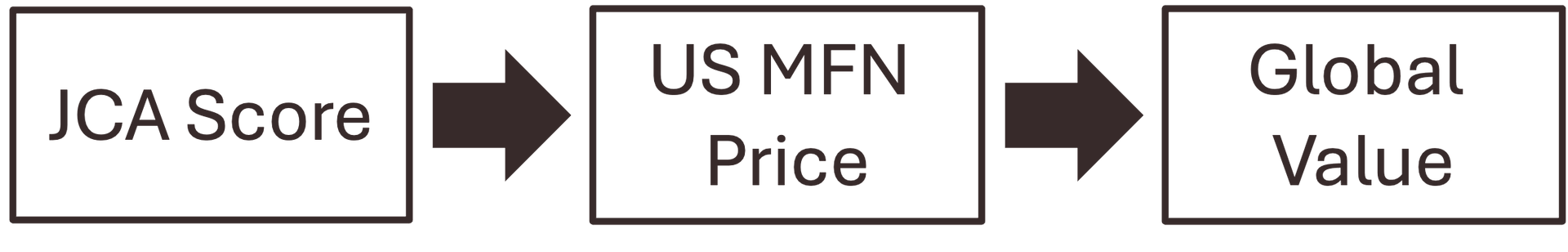
We Bridge the McKinsey Gap
Generalist consultants are experts at building rNPV models based on the assumptions you provide. We are the specialists who validate those assumptions. We audit the clinical architecture—the PICO alignment and the Evidence Quality—to determine if the "blockbuster price" in your spreadsheet is anchored in reality or if it is a valuation liability.
- Our Objective: Ensure your oncology or rare disease asset is positioned for a "High Added Benefit" rating under the JCA. Anything less is an immediate price anchor that limits your exit multiple.
- Preventing "European Contagion": By securing a robust evidence grade in the EU-4, we protect the US net price floor from the benchmarking effects of the 2025 MFN Executive Order.
Global Investor Intelligence & Strategy
We replace generalist assumptions with technical certainty across the clinical evidence lifecycle:
Strategic Execution: Switzerland & Beyond
While our strategy is global, our execution is local. We leverage deep expertise in premium launch markets to set strategic value anchors.
- Swiss Premium Access: Mastering the world’s most sophisticated semi-confidential P&R environment to set a strong global reference price.
- CEE Gateway Strategy: Utilizing local Croatian expertise to set regional precedents and optimize budget impact for Eastern European entry.
Our Partners Trust Our Approach




